
What You Need to Know About Omalizumab (Xolair) for Food Allergies
- On February 16th 2024, omalizumab was approved for the treatment of food allergies in select patients.
- It is indicated for the reduction of allergic reactions (Type I), including anaphylaxis, that may occur with accidental exposure to one or more foods in adult and pediatric patients aged 1 year and older with IgE-mediated food allergy.
- It is to be used in conjunction with food allergen avoidance.
How does it work?
- Omalizumab is a sub-cutaneous injection, 1-2 injections will be administered every 2-4 weeks (based on patient’s weight and total IgE level)
- Omalizumab binds free IgE in the blood, which helps decrease the body’s ability to mount an allergic reaction.
Is it safe?
- Omalizumab has been approved for the treatment of asthma since 2003.
- It was approved to treat asthma in children over 6 years old since 2016.
- It has also been approved for the treatment of urticaria (hives), and nasal polyps.
- There is a risk of anaphylaxis to omalizumab. This can occur in 0.1% of patients.
- There were no incidents of anaphylaxis to omalizumab in the food allergy study.
- The most common side effect is injection site reaction.
- “Some concerns about omalizumab safety have been raised as its use has been recently linked to potential increased cancer risk. Nevertheless, literature evidence does not support this statement, and clinical studies and evidence from real-world registries and surveillance analysis have consistently reported drug safety.” – Bagnasco, D. World Allergy Organization Journal (11/2022).
- 1 patient (1 year of age) in the food allergy study was found to have elevated liver enzymes, however after complete evaluation this was felt to be unrelated to omalizumab.
Study details
- The OUtMATCH trial is a 3 part study to evaluate the safety and efficacy of omalizumab as a treatment that blocks immune responses irrespective of antigen type for patients as young as 1 year of age who are allergic to multiple foods.
- OUtMATCH stands for Omalizumab as Monotherapy and as Adjunct Therapy to Multi-Allergen Oral Immunotherapy (OIT) in Food Allergic Children and Adults.
- Part 1 was published February 25th 2024 and is the supporting evidence for the current omalizumab indication for food allergy.
- Part 2, still ongoing, will investigate the use of omalizumab in addition to OIT (oral immunotherapy).
- Part 3, still ongoing, will investigate allergen tolerance after discontinuation of omalizumab or OIT.
OUtMATCH Part 1 Results
After 16 weeks of omalizumab treatment:
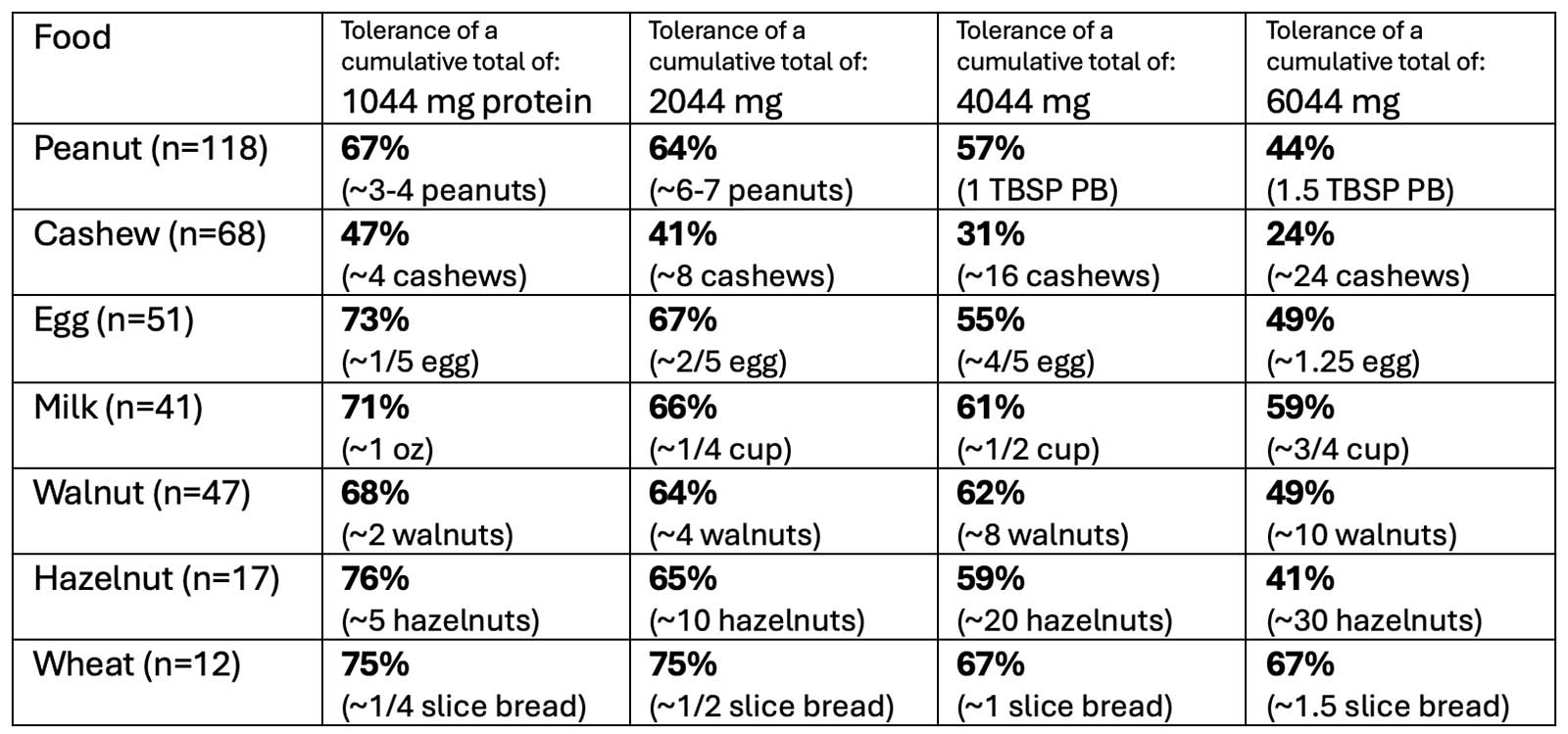
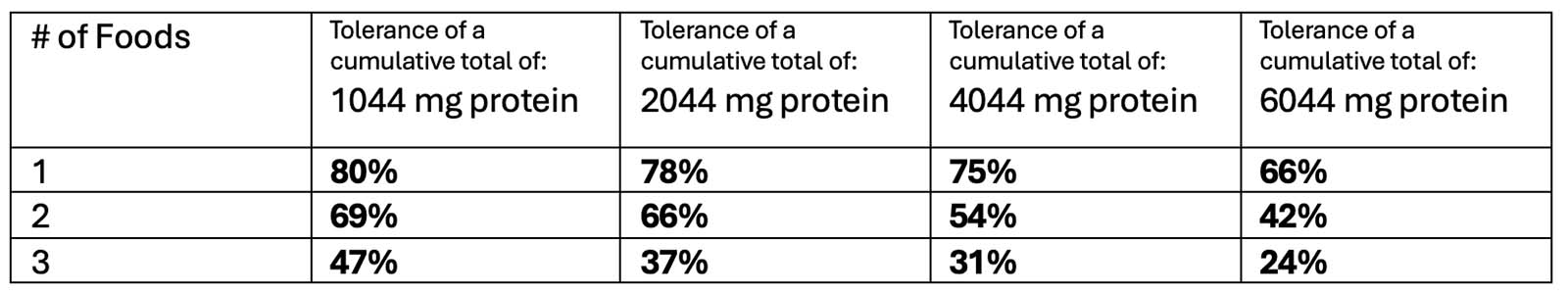
*TO NOTE: 14% of participants could NOT consume >30 mg of peanut protein (10% of a peanut)
